Device for intradermal
injection of vaccines
(in development)
Device for intradermal administration of vaccines
Lightnix aims to solve the social challenges surrounding vaccines and contribute to global medical innovation for infectious disease prevention by using our novel drug intradermal administration device inspired by mosquito needles.
Lightnix is developing
novel devices for drug
intradermal
administration

Lightnix intradermal administration device
Our goal is to create devices that enable simple, accurate, stable administration of vaccines into the intradermal area where many antigen-presenting cells gather.

Concept
- Intradermal (ID) administration devices that show equivalent efficacy to conventional intramuscular administration with less vaccine.
- ID administration, which was considered difficult, can be performed by simply pushing our devices onto the skin like a stamp.
- An innovative structure that applies the original technology “PINNIX Light,” the world’s first biodegradable lancet device.
Scientific information on intradermal vaccine administration can be found here.
SOCIAL ISSUESIssues Related to Global Vaccines
Many social problems regarding infectious disease prevention remain in the world.
In human history, vaccines can be said to be the most life-saving drugs. However, the global supply is still insufficient due to the high demand, and various challenges have been pointed out.
Current situation in developing countries
Developing countries cannot receive sufficient vaccine supply due to the amount of vaccine supply and economic situation. As a result, there is a gap in infectious disease prevention between developed and developing countries.
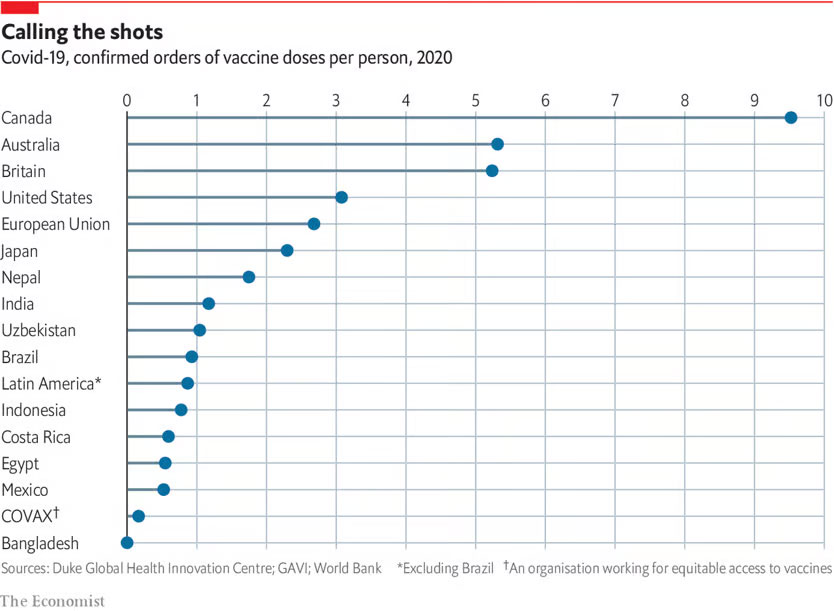
Source: The Economist, Rich countries grab half of projected COVID-19 vaccine supply, 2020.
Of the causes of death for 0-4 years old children in developing countries, 40% are treatable diseases in developed countries.
This is because vaccines are expensive and only limited vaccines are available in developing countries.
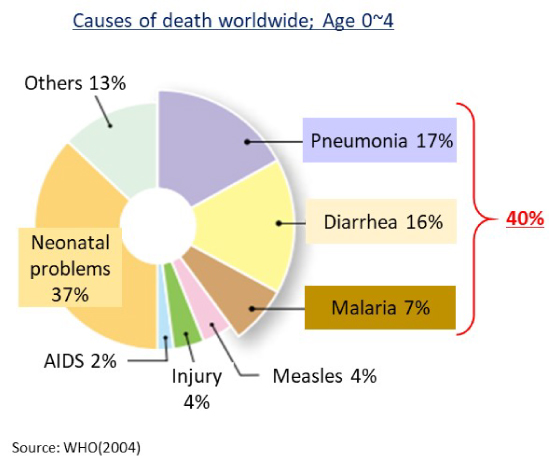
Source: Modified from Global Under-5 Mortality Factors, WHO, 2004.
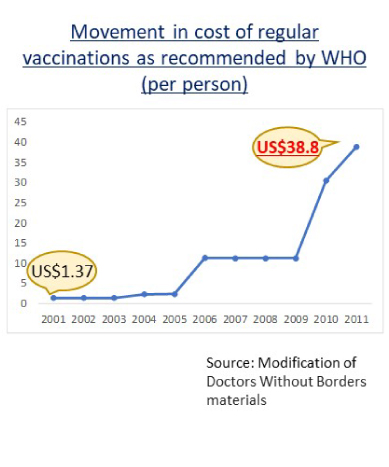
Source: Modified from Movement in cost of regular vaccinations as recommended by WHO (per person), Doctors Without Borders
Moreover, to reduce the purchase cost of vaccines in developing countries, vaccines are supplied in the form of Multi-dose Vials, which contain multiple doses of vaccines for many types of vaccines.
However, the amount of vaccine wasted without being used up in Multi-dose Vials is an issue.
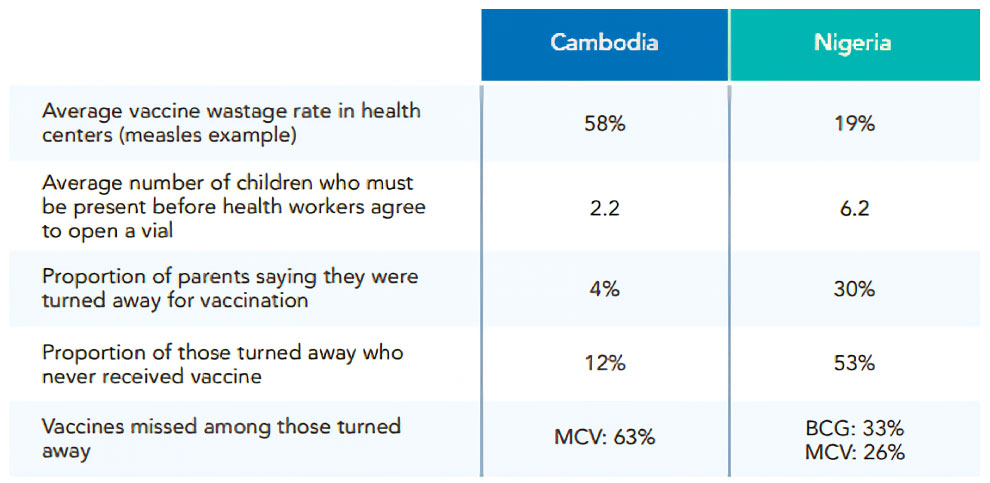
Source: DCVMN, Vaccine Wastage Rate in Cambodia and Nigeria
Therefore, there is a demand for the supply of single-dose vaccines, which are individually packaged for each use and cheaper than the current ones.
Shortage of medical workers able to administer vaccines
When it is necessary to simultaneously administer vaccines to several people, as in the case of a pandemic, the shortage of medical workers to administer vaccines becomes an issue.
The burden of advance preparation, such as vaccine preparation, also requires simple administration devices that require little advance preparation.
Furthermore, there are several vaccine administration methods, such as intramuscular, subcutaneous, and intradermal injections. However, a method that requires high injection techniques, such as intradermal injection, limits the number of medical personnel who can administer the vaccine.
This is one of the reasons for the bottleneck in the global vaccine dissemination.
Environmental problems
Disposal of syringes after use is also a major issue in developing countries.
Illegal dumping, environmental contamination by medical needles that cannot be incinerated, and the risk of secondary infection due to needle stick accidents form a serious medical problem.
A global WHO survey in February 2022 reported 731,000 L of chemical waste, including vaccine drugs, 144,000 t of solid waste, including syringes and needles, and a 10-20% incidence of needlestick during waste collection.
Tonnes of COVID-19 health care waste expose urgent need to improve waste management systems, WHO; 2022.
https://www.who.int/news/item/01-02-2022-tonnes-of-covid-19-health-care-waste-expose-urgent-need-to-improve-waste-management-systems
Conventional syringes carry the risk of secondary infection due to contaminated needles. Despite ongoing efforts to develop needle-free devices, many issues remain unresolved in the development of a vaccine administration device that can fundamentally mitigate the spread of vaccines in developing countries, such as low cost and materials that can be incinerated after use.
SCIENCEAdvantages of Intradermal Vaccines and Clinical
Vaccine supply shortage and purchase costs have been discussed globally. To achieve the same efficacy while reducing the cost of use, research and clinical applications of intradermal vaccine have been conducted as an administration method.
About intradermal vaccination
Vaccine injection methods are divided into intramuscular, subcutaneous, and intradermal administration. However, many studies have demonstrated that intradermal administration has significant advantages over other methods.
The intradermal area represents the extremely shallow layers of skin between the epidermis and dermis, where immune cells that contribute to immune responses are densely localized.
Therefore, WHO has reported that intradermal administration has higher immunogenic potential than intramuscular or subcutaneous administration, which are the main conventional administration routes.
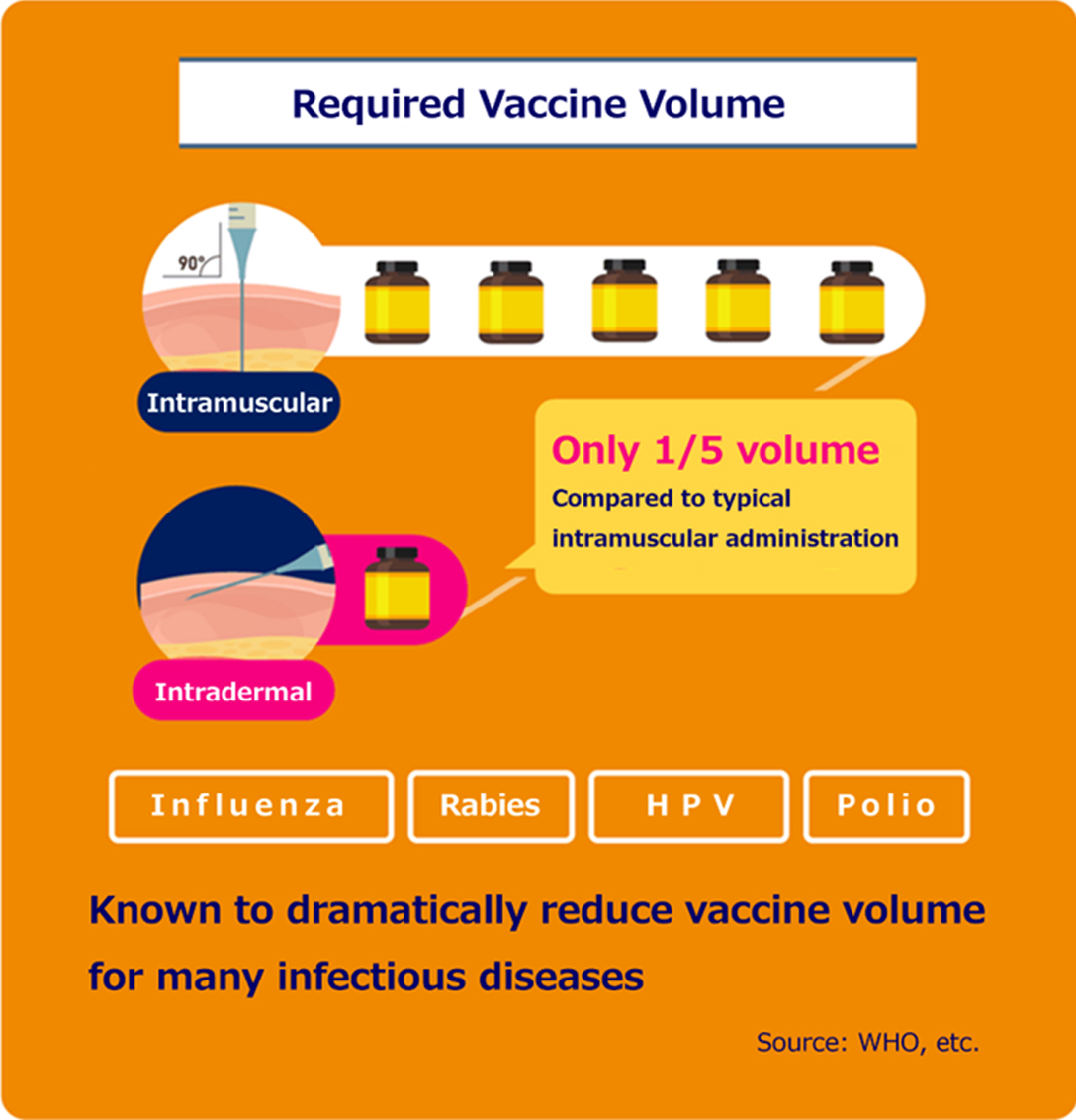
According to reports, the intradermal vaccine administration is sufficient to achieve immunity even if the dose is reduced to 1/5 of that of the intramuscular administration.
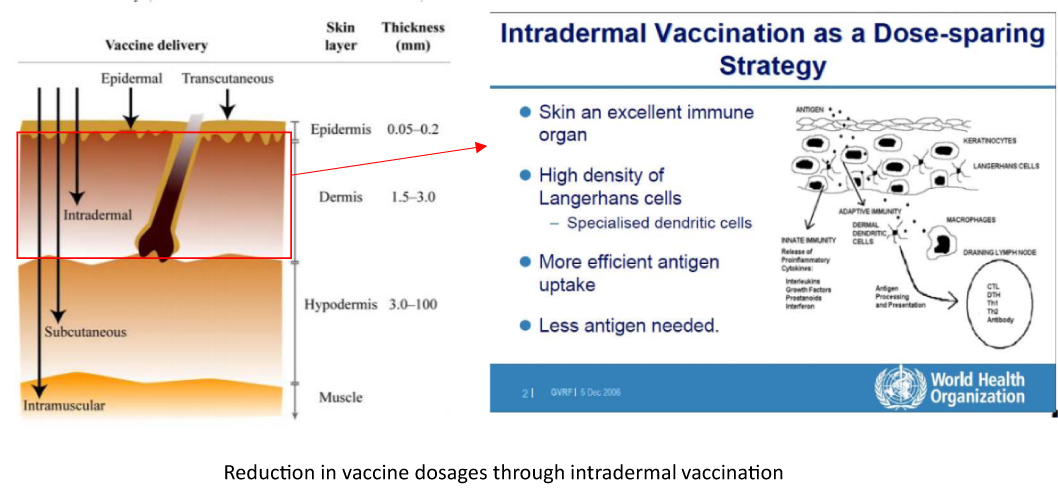
Intradermal administration is expected to reduce the amount of vaccine because it can be administered locally to the site where immune cells congregate.
Source: Intradermal Delivery of Vaccines Report, WHO; 2009. And modified from Global Vaccine & Immunization Research Forum, WHO; 2006.
The Thai Red Cross has developed an intradermal rabies vaccine administration regimen that has reduced vaccine doses by 80% (Vaccine. 2006 Apr 12;24(16):3084-6.).
This regimen has the historical background of being recommended to many countries by WHO as a TRC-ID regimen.
The clinical benefits of intradermal administration of influenza and many other vaccines have also been reported to reduce vaccine doses and are attracting attention (N Engl J Med. 2004 Nov 25;351(22):2295-301.)
| Immunogenicity of influenza vaccines 42 days after administration |
IM 15 μg (N=50) |
ID 3 μg (N=50) |
|
|---|---|---|---|
| Fold increase in GMT (Fold) |
H1N1 | 12.7 | 13.4 |
| N3N2 | 6.0 | 16.4 | |
| B | 13.1 | 10.4 | |
| Seroconversion rate (%) |
H1N1 | 74 | 84 |
| N3N2 | 60 | 78 | |
| B | 78 | 72 | |
| Seroprotection rate (%) |
H1N1 | 90 | 86 |
| N3N2 | 94 | 96 | |
| B | 100 | 92 | |
Modified from New England Journal of Medicine, Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM, Dose sparing with intradermal injection of influenza vaccine, 351(22), 2295-301. Copyright © 2004 Massachusetts Medical Society.
Mechanisms of immune activation by intradermal vaccination
Research is indicating that the immunological mechanisms, which are specific to cutaneous function governing the barrier between the inside and outside of the body from foreign and infectious sources, are the reasons for the high immunogenic potential of intradermal administration.
《Topics》Mechanism of immune induction by intradermal administration
Structure of the skin and localization of immune cells
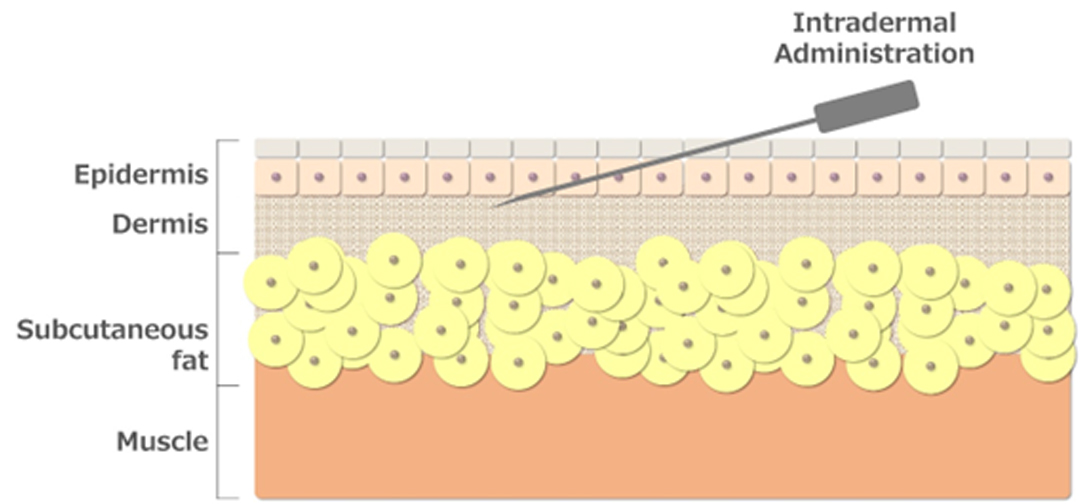
The skin structure consists of the epidermis, dermis, subcutaneous fat, and muscle, of which the intradermal region is anatomically defined as the interior of the epidermis and dermis.
Vaccine injections include intramuscular, subcutaneous, and intradermal injections.
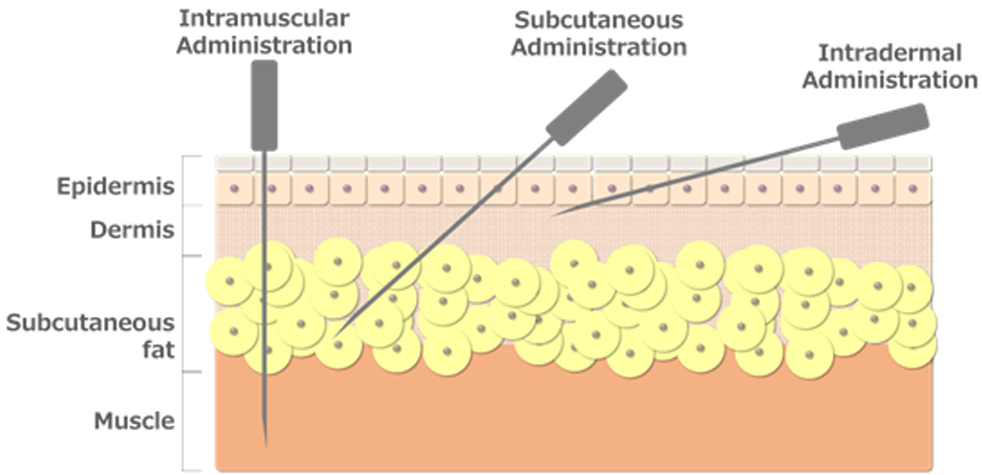
We are aiming for widespread use of intradermal injections because it is related to the density of immune cells in the skin.
The skin structure consists of the epidermis, which is about 0.2 mm thick and comprises four layers from top to bottom: stratum corneum, stratum granulosum, stratum spinosum, and stratum basale; the papillary layer, which is attached to the stratum basale; and the dermis, which is about 1 to 2 mm thick and entails a network of layers spread out below the papillary layer.
Compared to intramuscular or subcutaneous injection, intradermal injection induces highly efficient immune activation even with a small dose of vaccine because many antigen-presenting cells are localized in this area, which is only 2 mm from the body surface.
Being only 0.2 mm in size, the epidermis is highly concentrated in antigen-presenting Langerhans cells, which are key components of the immune system against invasion of foreign substances on the skin.
The dermis lies beneath the epidermis and contains many antigen-presenting dendritic cells and macrophages.
The dermis mostly comprises a network of fibrous proteins, which consist of collagen, elastin, and reticulin fibers. Dendritic cells and macrophages are localized in the area.
Dendritic cells and macrophages are distributed in subcutaneous tissues and muscles. However, they are localized particularly densely in the dermis and play the roles of phagocytosis and degradation of foreign substances and antigen-presenting cells in skin immunity.
These skin-localized antigen-presenting cells take in foreign substances, such as bacteria, viruses, and allergens, which invade the epidermis and dermis from the body surface. By exposing molecules specific to some foreign substances to the cell membrane surface, they activate other immune cells such as T cells, and immunity is acquired to eliminate the foreign substances.
Immune responses by Langerhans cells in the epidermis and dendritic cells and macrophages in the dermis
Immune responses by Langerhans cells in the epidermis and dendritic cells and macrophages in the dermis.
First, macrophages localized below the dermis play a crucial role in cellular immunity by phagocytosing and degrading foreign substances. After being activated by foreign substances or viral infection, they secrete cytokines that mobilize other phagocytic cells, such as neutrophils and natural killer cells (NK cells), to promote immune cell aggregation. That is, they stay in the affected area and phagocytose foreign substances while producing inflammation.
In addition, by presenting some of the phagocytosed molecules as antigens on the cell membrane surface, they are recognized by helper T cells (Th cells). These Th cells are the command tower of antibody production and are the starting point of humoral immunity. They promote antibody production by activating antibody-producing B cells and elimination of foreign substances with antibodies.
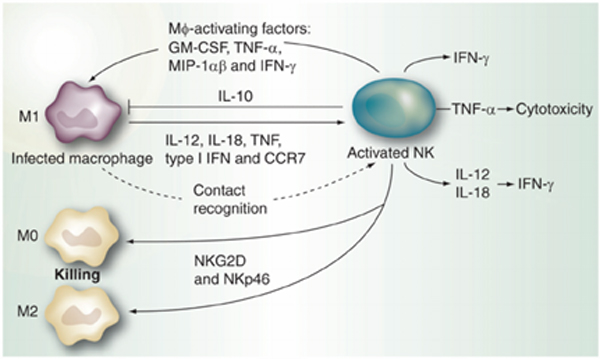
Source: Malhotra A, Shanker A. NK cells: immune cross-talk and therapeutic implications. Immunotherapy. 2011 Oct;3(10):1143-66. doi: 10.2217/imt.11.102. PMID: 21995569; PMCID: PMC3230271.
In contrast, after phagocytosis of foreign substances, unlike macrophages, Langerhans cells and dermal dendritic cells localized in the epidermis migrate to neighboring lymph nodes.
Dendritic cells that have ingested foreign substances migrate to lymphatic vessels that run below the dermis, take up lymphatic vessels, and migrate to lymph nodes. Dendritic cells migrate into the lymph node and present antigens on the surface of the cell membrane to activate Th cells, which are the command center of immune activation, inducing antibody production by B cells and killing infected cells by NK cells and cytotoxic T cells (CTL), which are potent cytotoxic cells.
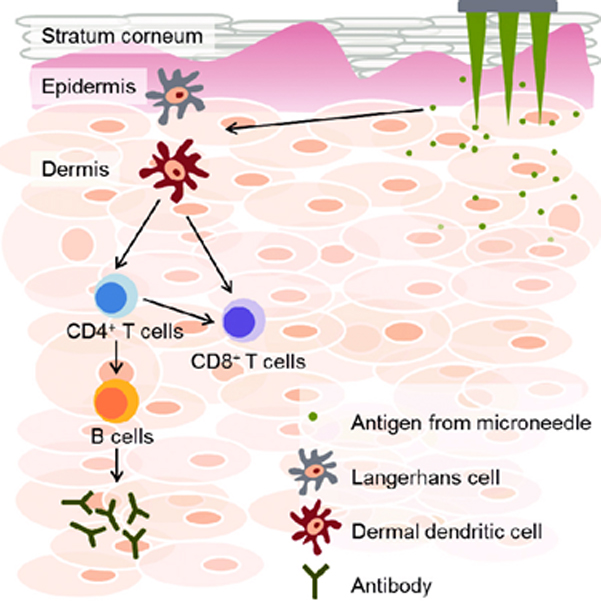
Source: Kwon KM, Lim SM, Choi S, Kim DH, Jin HE, Jee G, Hong KJ, Kim JY. Microneedles: quick and easy delivery methods of vaccines. Clin Exp Vaccine Res. 2017 Jul;6(2):156-159. doi: 10.7774/cevr.2017.6.2.156. Epub 2017 Jul 26. PMID: 28775980; PMCID: PMC5540964.
Another major difference from macrophages is that they are incapable of activating immature T cells. In contrast, Langerhans cells and dendritic cells can induce differentiation from immature naive T cells that have never accepted an antigen to mature effector T cells with immune functions.
The immunity induced by macrophages and Langerhans /dendritic cells differ.
Macrophages are primarily responsible for cellular immunity and are less capable of inducing antibody production than dendritic cells.
On the other hand, Langerhans cells and dendritic cells can induce differentiation of naive T cells into various types of effector T cells, which is a strong starting point for both cellular and humoral immunity and can induce more potent antibody production than macrophages. Langerhans cells can take up antigens at the protein level outside the skin. In contrast, dermal dendritic cells take up antigens that enter the dermis and present them.
Therefore, compared to intramuscular or subcutaneous injections, in which macrophages acquire immunity mainly through macrophages, intradermal administration, in which macrophages are more densely localized and dendritic cells have strong immune activation ability, is suggested to be more efficient in vaccine-induced immune acquisition.
Technical issues of intradermal vaccination
As described above, intradermal administration is expected to improve the efficiency of immune acquisition. However, the difficulty of the procedure has hindered its widespread use.
The intradermal injection method targets an exceedingly shallow area of the skin (1-2 mm from the epidermis).
This requires a high level of technique, with the needle tilted almost horizontally to the skin at a 10° angle.
Compared to other methods, such as intramuscular or subcutaneous injections, intradermal injections have been studied for their high immunogenicity compared to other methods. However, the fact that only a limited number of medical professionals can perform them due to the high level of skill required has become a bottleneck in their widespread use.
Therefore, an administration device for intradermal injection that is simple and reproducible is needed.
PRODUCTSProposal for Intradermal Administration Devices
Focusing on reducing vaccine dosage and various other advantages of intradermal administration, we are developing a simple and reliable intradermal administration device to enhance the widespread use of intradermal administration.
Concepts of our device
We aim to improve and spread vaccine immunization by developing intradermal vaccine administration devices.

Lightnix Intradermal Administration Device
- Intradermal (ID) administration devices show equivalent efficacy to conventional intramuscular administration with vaccine.
- ID administration, which was generally considered difficult, can be performed by simply pushing our devices onto the skin like a stamp.
- An innovative structure that applies the original technology, “PINNIX Light,” which is the world’s first biodegradable lancet device.
We aim to develop devices that can be easily and reproducibly administered by pressing them like a stamp, eliminating the need for delicate needle tip angle adjustment.
In addition, the risks of secondary infection due to needlestick accidents after disposal of conventional metal needles have been a severe problem.
Due to needlestick accidents after disposal, conventional metal needles have a serious risk of secondary infection. However, by adopting an automatic needle tip retractor mechanism based on the PINNIXLight® lancet device technology developed by our company, we aim to eliminate the risk of secondary infection caused by medical waste and realize safe medical care.
It is inexpensive because the housing is almost entirely made of plastic or biodegradable resin. We aim for a supply system that can be distributed in sterilized, individually packaged form.
This will enable the distribution and self-administration of pre-filled vaccine devices from medical institutions to households, , thereby dramatically promoting the spread of vaccines in developing countries in the future.
Results of immunological experiments on our device
We had also been selected by the Japan Agency of Medical Research and Development, AMED project, to begin developing the device in 2019.
Currently in transition from pre-clinical to clinical stage and seeking collaboration with pharmaceutical companies.
In vivo experiments using rats, egg albumin (OVA), which is commonly used as a model antigen, was administered to the rats by the prototype device. The results demonstrated that the serum antibody titer (IgG Titer) was equivalent to that given through muscle injection, even though the vaccine volume was 1/5 of that given through intramuscular administration.
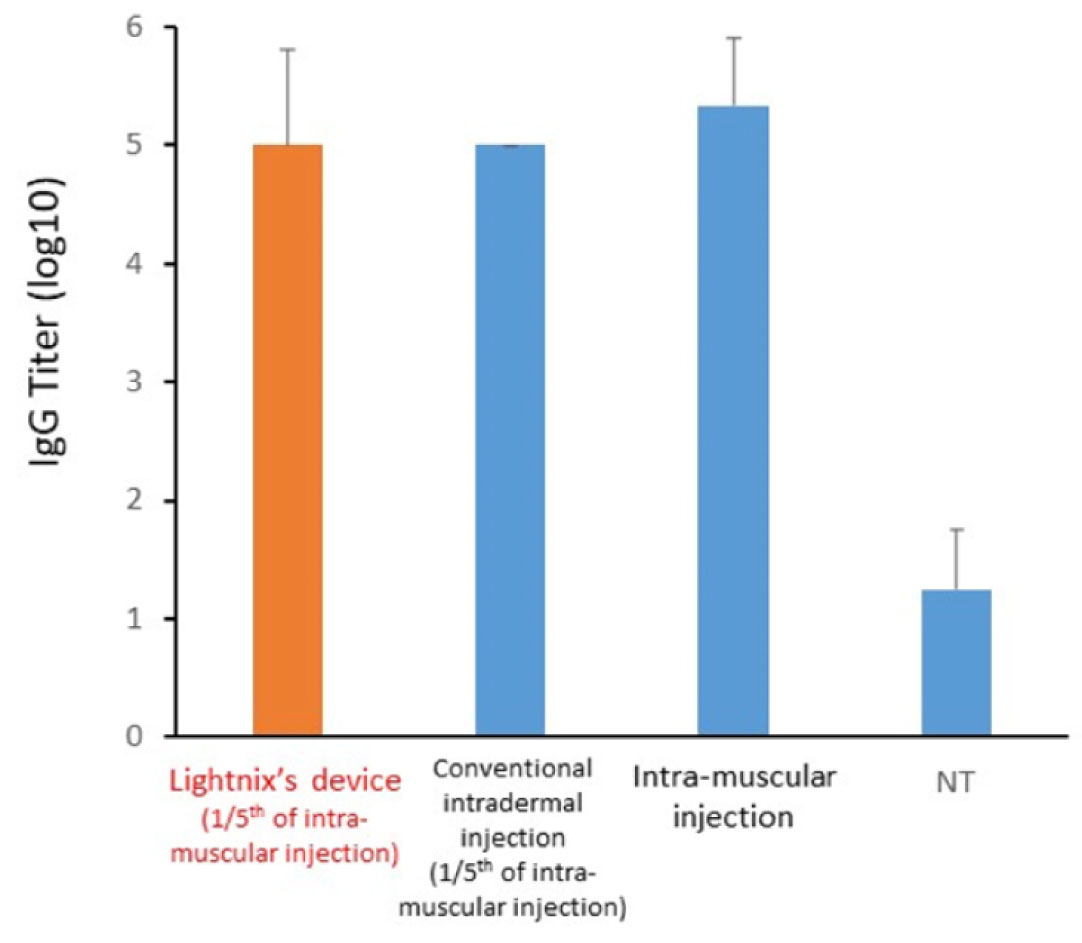
Results of OVA administration to rats by our vaccine intradermal administration device (prototype).
The results of intradermal administration of 1/5 of the vaccine volume of intramuscular administration by our device showed an increase in antibody titer equivalent to that of intramuscular administration.
In addition, because the influenza virus is zoonotic, our device was effective in reducing vaccine volume in an experiment in which the influenza vaccine was administered to ferrets, commonly used as model animals for influenza infections.
Compared to intramuscular administration, even a vaccine volume of 1/5 was found to be sufficient for acquiring immunity, thereby demonstrating the effectiveness of reducing the vaccine volume administered.
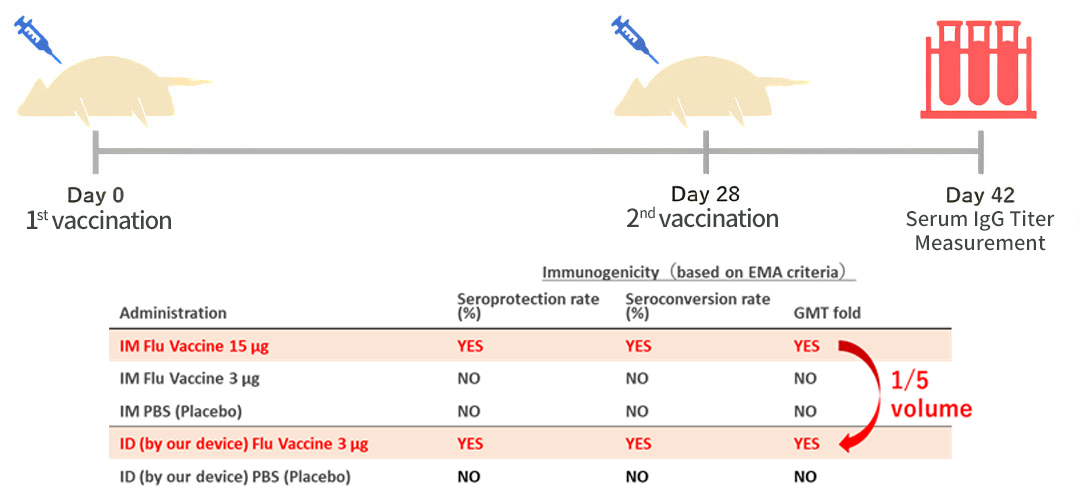
Immunogenicity evaluation of influenza vaccine two weeks after administration to live ferrets
All the three items demonstrated immunogenicity by our ID device according to EMA criteria with 1/5 volume of intramuscular administration.
While the EMA requires that any of the three criteria for evaluating influenza vaccines be immunogenic, our device was immunogenic for all three criteria.
The results of the above experiments were conducted under the support of a project adopted by AMED (Japan Agency for Medical Research and Development), which demonstrated the effectiveness of intradermal administration using our device in reducing vaccine doses.
Various other pharmacological benefits
In addition to reducing vaccine dosage, intradermal administration has been suggested to have various pharmacological advantages.
The vaccine is less likely to circulate throughout the entire bloodstream because blood vessels travel less intradermally than in the muscles. The administered vaccine is more likely to be localized to the site of administration. Adverse reactions are more likely to be localized rather than systemic.
Even for those with a constitution that does not respond to intramuscular injections*, switching to intradermal injections can suggest the acquisition of immunity.
(※ Non-responder group
For example, it has been suggested that HIV-affected patients are less likely to be induced to acquire immunity by common hepatitis B vaccination. At the same time, intradermal injection improves vaccine-induced immunity.
Source: Yoshimura Y, Sasaki H, Miyata N, Tachikawa N. Intradermal Hepatitis B Vaccination in Non-responder People Living with Human Immunodeficiency Virus in Japan. Jpn J Infect Dis. 2022 Sep 22;75(5):519-522. doi: 10.7883/yoken.JJID.2021.834. Epub 2022 Apr 28. PMID: 35491226.
Source: Filippelli M, Lionetti E, Gennaro A, Lanzafame A, Arrigo T, Salpietro C, La Rosa M, Leonardi S. Hepatitis B vaccine by intradermal route in non responder patients: an update. World J Gastroenterol. 2014 Aug 14;20(30):10383-94. doi: 10.3748/wjg.v20.i30.10383. PMID: 25132754; PMCID: PMC4130845.
In addition to the pharmacological benefits described above, research on intradermal administration of vaccines is a method that is anticipated to generate many more reports in the future.
PURPOSEThe Future Realized by Lightnix
Medical innovations in infectious disease prevention
We will provide simple and inexpensive intradermal administration devices.
Our devices will provide the pharmacological benefits of intradermal administration, such as reduced vaccine volume and adverse events. Moreover, in the prevention of a wide range of infectious diseases, they will be developed and medically innovated to achieve the following.
Enable more people to access vaccine supply during pandemics, when vaccines are in short supply due to sudden outbreaks.
Increase global vaccination coverage, including in developing countries, by reducing the cost of purchasing vaccines.
Enable safer vaccination by reducing the risk of systemic and serious adverse reactions.
Enable more efficient immunization against vaccines that are difficult to immunize with intramuscular injections.
Dramatically increase vaccine coverage in developing countries by distributing pre-filled vaccine devices to each household to enable self-administration













